 Skin Relief Moisturizing Lotion
Skin Relief Moisturizing Lotion 
AVEENO® SAMPLES FOR YOUR PATIENTS
Receive news on the latest skincare innovations, sample opportunities, hot topics in literature, patient insights and resources for your patients.
Results of a 9-week study showed the efficacy of AVEENO® Skin Relief Lotions and the impact of patient preference in improving patient compliance:
9 out of 10 patients stayed with AVEENO®
Both lotions were highly effective in relieving itchy, extra-dry skin
- Patients chose to apply the product more often than required, leading to greater improvement in skin parameters
- Given the choice of whether or not to continue with AVEENO®, 9 out of 10 stayed with AVEENO®
Clinical trial to determine compliance to and efficacy of AVEENO® Skin Relief Gentle Scent™ vs. fragrance-free lotions in subjects with moderate to severe itchy, dry skin1
Objective:
To evaluate the impact of usage rates of a scented versus a fragrance-free body lotion on patient compliance and determine whether increased lotion usage leads to improved efficacy for parameters of moisturization and itch.
Study Design:
Sixty-one females, between the ages of 18 and 60, with moderate to severe dry skin with mild to moderate itch (Fitzpatrick Skin Types I-IV) completed this single-center, evaluator blinded, randomized 9-week clinical study. Test products included: AVEENO SKIN RELIEF GENTLE SCENT® Moisturizing Lotion (Coconut) and AVEENO® Skin Relief Fragrance Free Moisturizing Lotion (Unscented).
Subjects were assigned AVEENO® Skin Relief Fragrance Free Body Wash in place of their normal body cleanser to use throughout the 9-week study period.
The 9-week study period was split up into 3 phases of product usage:
- Phase 1 (Weeks 0 to 3): Subjects were given a body lotion (Study product) to apply on legs and body twice daily.
- Phase 2 (Weeks 3 to 6): Subjects were instructed to use the same study product as in phase 1, however, with no fixed regimen (subjects can apply as often as they wished).
- Phase 3 (Weeks 6 to 9): Subjects were allowed to either continue using the study product, or switch to any other marketed lotion, with no fixed usage regimen (subjects can apply as often as they wished).
Efficacy Measures:
- Clinical Efficacy Grading
- Skicon 200EX Measurements
- Subject Self-Assessment Questionnaires-end of all phases Final Self-Assessment Questionnaires
Results:
Clinical Evaluations:
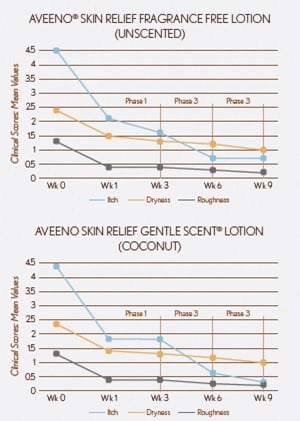
Clinical parameters of itching, dryness, roughness and scaling showed significant improvements in Phase 1 (week 3 vs baseline - week 0) for both lotions.
Significant additional improvements in itch, dryness, roughness and scaling were observed when comparing Phase 3 to Phase 1 for both lotions.
Results showed significant percentage increases in mean application when comparing Phases 2 & 3 to Phase 1. Subjects applied more often during these phases (by choice), leading to greater improvement in clinical parameters.
SKICON measurements showed significant increases in moisturization in Phase 1 (week 0 vs week 3).
Self Evaluations:
Subjects perceived significant improvements for all parameters in Phase 1 (week 3 vs baseline - week 0).
Significant improvements in various individual parameters were perceived during Phase 2 (week 6 vs week 3) and Phase 3 (week 9 vs week 3) when compared to Phase 1 data for both the scented and unscented products.
8.1_figure_1.jpg





All Fields required, unless otherwise indicated
Will be used as your user name
By submitting your information above, you agree that the information you provide will be governed by our site's Privacy Policy.