A clinical evaluation of the tolerability of a seaweed extract containing shampoo on patients with atopic eczema, seborrheic dermatitis or rosacea
Kristine Schmalenberg, Ph.D.*, Joseph F. Fowler Jr. MD, FAAD
*Johnson & Johnson Consumer & Personal Products Worldwide, Skillman, New Jersey
Abstract
Patients with sensitive skin conditions such as atopic eczema, seborrheic dermatitis or rosacea often present with irritation from personal care products such as shampoos. In addition to their desired beneficial effects, cleansing products can cause unwanted damage to the skin. There are many ways in which a cleanser interacts with the stratum corneum including removing skin components, such as lipids and NMF, as well as remaining in the skin after rinsing and disrupting the lipid order.
In this study, a shampoo with NATURASURF™ - a new naturally derived gentle cleansing technology and Balancing Seaweed Extract, an ingredient that has been scientifically proven to provide balanced cleansing to ensure that hair isn’t over-stripped of its natural moisture, was tested on thirty-four subjects with either atopic dermatitis, seborrheic dermatitis, or rosacea. Patients were selected from the practice of the investigator and were supplied with the shampoo test product. Subject and investigator assessments were performed at baseline and weeks 1, 2, and 3.
In the opinion of the investigator, the test product was very well tolerated by patients with rosacea, atopic dermatitis, and/ or seborrheic dermatitis and may be useful as an adjunct in the management of these conditions.
Study Design
Objective
To determine if a rinse-off personal care shampoo and conditioner product are well tolerated with patients diagnosed with atopic eczema, seborrheic dermatitis or rosacea.
Design
This was a controlled one cell study; subjects used the product regimen at least 5 times a week over the study duration. Clinical evaluations were made at baseline, week 1, week 2 and week 3. Patient’s skin and scalp were assessed and graded by the investigator for Objective Parameters including Erythema, Edema and Scaling, made on a 4-point scale as follows, 0 = none, 1 = mild, 2 = moderate, 3 = severe. In addition, during the same visits, patients were asked to rate Subjective Parameters including Itching, Dryness/Tightness, Burning, Tingling and Stinging according to the same 4-point scale.
Population
The study population consisted of male and female volunteers who had a diagnosis of atopic eczema, seborrheic dermatitis or rosacea, over 18 years old.
Results
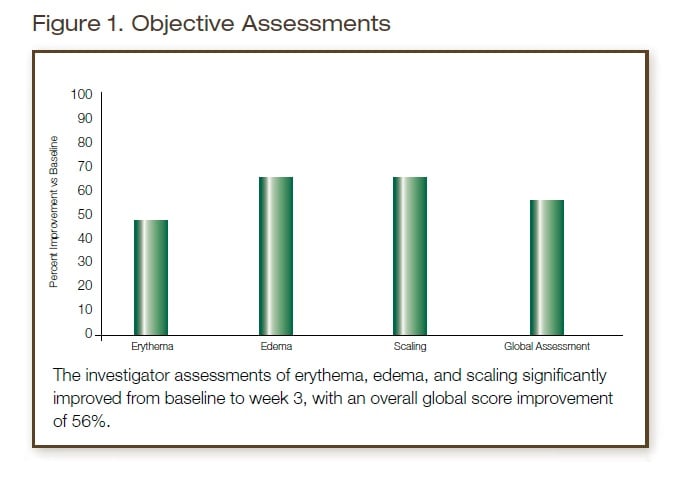
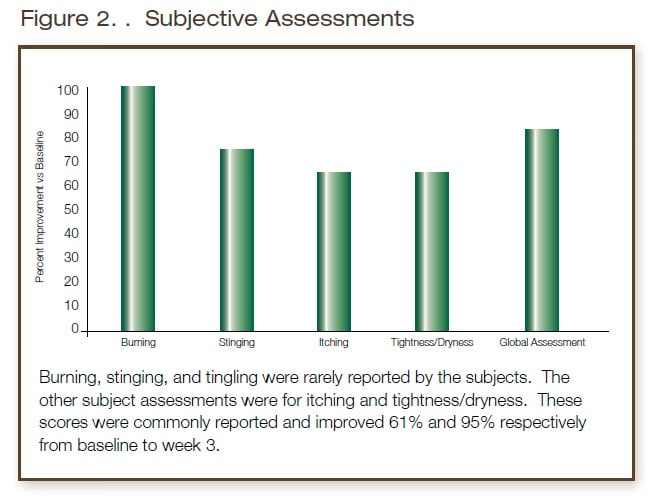
The following parameters were graded by an expert clinical grader on a 0 (none) to 3 (severe) scale: Erythema, Edema, Scaling; 0=none, 1=mild, 2=moderate, 3=severe. The patients’ skin and scalp were assessed and graded by the investigator and graded for objective and subjective irritation (erythema, edema, scaling, burning, stinging, tingling, itching, and tightness/dryness). The patients also kept a diary and were interviewed for any irritation or problems during use of the investigational products as well as any change of regimen, adverse experiences, concurrent illnesses and use of concurrent therapies/medications.
pure_renewal_graph_1.jpg
The primary outcome measure of this study was the proportion of patients experiencing any untoward effects on their atopic eczema, seborrheic dermatitis or rosacea that seemed to directly correlate (all confounders eliminated to the extent possible) to exposure to the shampoo and conditioner. Secondary outcome measures included measurement of proportion of irritant, urticarial or contact dermatitis reactions to each product tested.
30 subjects showed improvement in the investigator global severity scores. Two showed no change (subjects 27 and 32). Two showed slight worsening, subject 14: baseline 1.0, week |three 2.0 and subject 30: baseline 0.5, week three 1.0.
Of the 30 subjects with a total symptom score > 0 at baseline, (subject assessments) three showed worsening (subject 17: 1.5 to 2.5; subject 21: 0 to 0.5; subject 22: 0 to 1.0) and one (subject 28: 1.0 to 1.0) showed no change.
pure_renewal_graph_2.jpg
Conclusions
The shampoo test product was very well tolerated by the subjects. Significant improvement was seen in both investigator assessments and subject symptom scores overall. Only 2 subjects showed slight worsening by IGA scores and 3 showed slight increases in symptoms by subject assessment. These low numbers are consistent with the natural course of the conditions studied. No adverse effects or intolerance attributable to the test product were noted. In the opinion of the investigator, the test product was well tolerated by patients with rosacea, atopic dermatitis, and/or seborrheic dermatitis and may be useful as an adjunct in the management of these conditions.
Acknowledgements
Hill Top Research, Winnipeg, Canada

All Fields required, unless otherwise indicated
Will be used as your user name
By submitting your information above, you agree that the information you provide will be governed by our site's Privacy Policy.